FDA Warnings To Pharma Caused Big Drop In Search Ads
The US Food & Drug Administration (FDA) issued warning letters issued in late March, 2009 to a number of big pharmaceutical companies, saying that their search ads were misleading and didn’t contain the required disclosure information mandated by US federal regulations. As one might have expected this caused the companies to temporarily pull their SEM […]
The US Food & Drug Administration (FDA) issued warning letters issued in late March, 2009 to a number of big pharmaceutical companies, saying that their search ads were misleading and didn’t contain the required disclosure information mandated by US federal regulations. As one might have expected this caused the companies to temporarily pull their SEM campaigns and resulted in a “59% drop in sponsored link exposures,” according to comScore:
An analysis of exposure to branded URLs within comScore’s data revealed that substantial declines occurred immediately following the letters being sent on March 26. Sponsored link exposures dropped 59 percent from 10.5 million during the week ending March 29 to 4.3 million during the week ending April 5. Declines in sponsored link exposures not only occurred in the weeks immediately following the letters, but continued over the next several months, plummeting 84 percent overall from March to June.
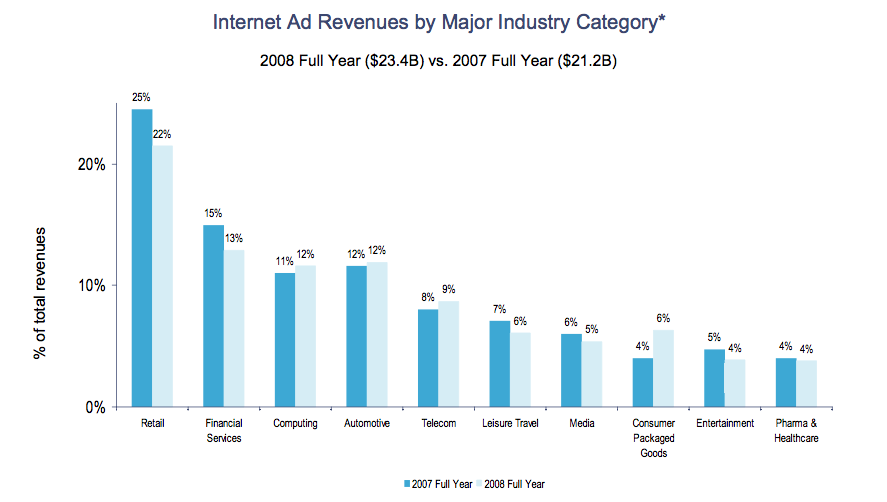
This basically means that they suspended their search ads while they tried to figure out how to comply with the FDA’s rules. Annually the pharmaceutical industry represents almost a billion dollars in online ad spending in the US. Here are the IAB’s numbers for 2008:
ComScore also said that organic sites promoting pharma products were also re-evaluated by the companies and experienced a traffic decline for a period of time:
Vanity and unbranded link exposures also experienced a decline, on average, across brands during the same period, although these methods were not under scrutiny in the FDA letters. Unbranded sites, which give additional information on the condition and treatment but do not directly promote the brand drug, declined 35-percent March to June to slightly more than one million exposures. Vanity URLs, which make no mention of a specific brand while generically describing a health condition but then redirect to the brand or drug’s website, declined 11 percent in June to 3.2 million average exposures versus March.
One of the challenges for the pharma industry in doing SEM for specific drugs is writing ad copy that is legally compliant, with a very limited amount of space in which to operate. It’s kind of absurd in a way. It thus may be that the industry will have to target conditions or ailments in paid-search ads and not mention specific drugs at all.
Contributing authors are invited to create content for Search Engine Land and are chosen for their expertise and contribution to the search community. Our contributors work under the oversight of the editorial staff and contributions are checked for quality and relevance to our readers. Search Engine Land is owned by Semrush. Contributor was not asked to make any direct or indirect mentions of Semrush. The opinions they express are their own.